“Pharma Plant War Stories” Spring Conference Delivers
- Posted by Howard Sneider
- On April 29, 2021
The Boston Area Chapter held their Virtual Spring Conference, Pharma Plant War Stories: Lessons Learned & Best Practices from Design to Operation, over the week of April 19-23. This virtual event was comprised of three tracks—Automation, CQV, and Design—and attendees had access to on-demand educational webinar sessions to watch on their own schedules. During the week, attendees participated in live 1-hour discussions with the speakers over Zoom, allowing for more in-depth conversations and questions.
The topics covered in this conference delved into the key ideas, strategies, and implementation processes that attendees could utilize in order to achieve the greatest value at their own facilities. This conference attracted a wide variety of attendees including Process & Automation Engineers, Quality Assurance, and Operations Professionals. The expert assembly of speakers provided new approaches to streamlining manufacturing processes and cost savings. This event provided Individuals or organizations involved in life sciences insights into new ways of thinking about existing challenges in life science manufacturing.
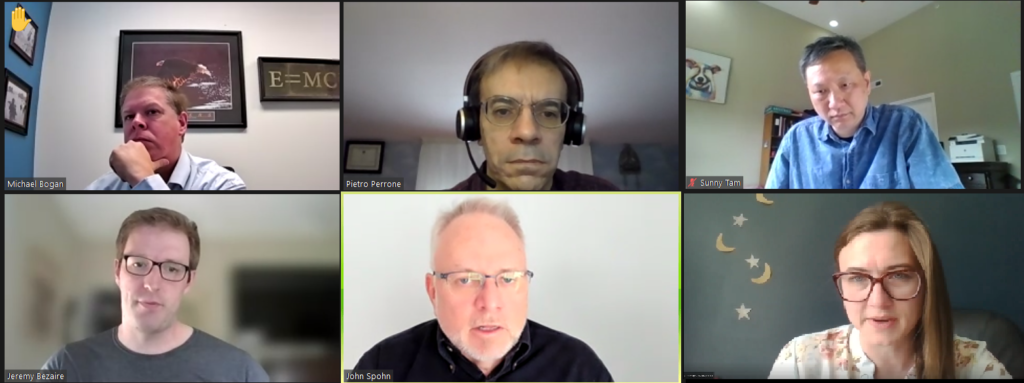
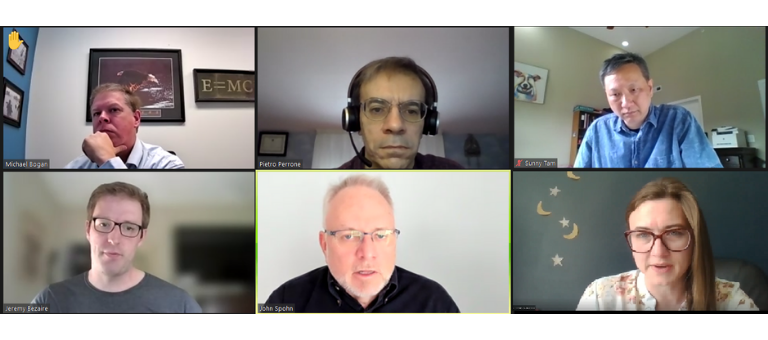
Attendees participate in an Automation Track discussion group for the session, “Advanced Automation for the Flexible Manufacture of Therapeutics” presented by Pietro Perrone, Chelsea Kuranda, and Jeremy Bezaire of Cytiva.
The Automation track featured presentations that highlighted implementations of advanced automation designs. The advances in automation discussed during the conference included discussions about the concept of a digital twin, or comprehensive computer model of the facility. The presentations also discussed a new approach to digital batch records where a bottom-up approach is easier to implement and faster than the traditional top down approach. There is a presentation on how artificial intelligence and machine learning can provide solutions that may be impossible to realize without the insight the applied algorithms provide. A presentation on connected manufacturing described how this approach seamlessly links unit operations physically and digitally across the entire process and permits discrete data to be complied into useful information and process knowledge.
The Commissioning, Qualification, and Validation (CQV) track provided a summary of the Commissioning & Qualification Baseline Guide Volume 5 – 2nd Edition by one of the contributors and described how changes in the past 20 years are making CQV more efficient and impactful. A presentation on the integration and interdependence of Factory Acceptance Testing, Start-up, and Commissioning echoed the approaches in the Baseline Guide and illustrated how careful planning can lower costs and shorten timelines while maintaining high control of quality. A presentation on QbD demonstrated how linking the patient and product needs to the facility design can help ensure that the completed facility will meet all the design requirements. This track also includes a presentation on a three-stage cleaning life cycle approach. This approach offers several advantages over a traditional cleaning validation.
The Design track offered a presentation that considers the important role that stainless steel systems play in a facility design, even when that facility has mostly or completely single use (SU) product contact surfaces. The track also included a presentation on strategies and best practices to follow when implementing facility renovations. The presentation looked at how existing, underutilized infrastructure can be leveraged to support new processes. The track also included a presentation on the implementation of HVAC and the energy savings that can be achieved by careful selection of operating designs.
The pre-recorded sessions are video recordings of the presentations by the conference guests with a small, superimposed postage stamp video of the presenter in the upper right corner. This format was selected by ISPE to allow the content to be delivered effectively in a remote setting and to allow the presenter to be seen so that they may enhance delivery of the content. The videos are hosted on our Conference Homebase site for on-demand viewing whenever you’d like – just contact office@ispeboston.org to register for access!
In general, the Q&A sessions were intimate events where attendees were able to gain additional insight from the presenters. Each of the sessions lasted about an hour. Since some of the presenters were joining the session from Europe, the Boston Area Chapter was very fortunate to have each presenter contribute to this important aspect of the conference. Video recordings of the Q&A sessions will be posted as well.
This event would not have been possible without the work of our Conference chair, Howard Sneider, and our track managers Mike Bogan and Catherine Stone. Our sincere thanks to our Platinum Conference sponsor Masy BioServices, our Gold Design Track sponsor Windover Construction, our Silver General Sponsor ICQ Consultants, and our conference supporter Kohler Co. Power Systems for supporting this event. And a huge thank you to our speakers and attendees for making this an awesome Spring Conference!



1 Comment